From June to August 2024, five partner companies of XtalPi (“QuantumPharm,” 2228.HK)—Leman Biotech, METiS Pharmaceuticals, Signet Therapeutics, META Pharmaceuticals, and ReviR Therapeutics—announced critical milestones, including IND approvals and significant funding rounds. These achievements validate the capabilities of XtalPi’s quantum physics-, AI-, and robotics-driven drug discovery platform, which supports both global pharma giants like Pfizer and Lilly and cutting-edge biotech innovators.
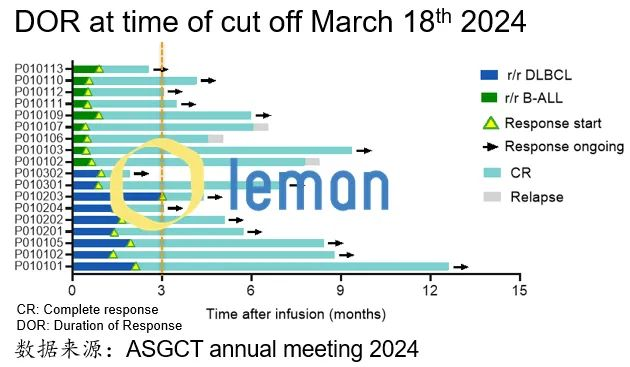
Leman Biotech: 50M RMB Raised, 100% Complete Remission in CAR-T Trials
- Funding: Completed 50M RMB angel+ rounds (total 150M RMB raised) to advance metabolism-enhanced CAR-T therapies.
- Milestone:
- 20+ adult/child patients achieved complete remission (CR) in IIT trials for CD19 CAR-T therapy.
- Doses reduced to 1% of commercial CAR-T levels, slashing costs and eliminating cytokine release syndrome risks.
- Tech Collaboration: Co-developed MetaAI-10 platform with XtalPi yielded 1,000+ ultra-high-affinity immune metabolic regulators (patent-pending), outperforming Stanford’s reported variants by 100x.
METiS Pharmaceuticals: $300M Total Funding, Leading AI-Driven Drug Delivery

- Funding: Closed $100M Series C (total $300M) to scale its AI platforms:
- AiLNP (RNA delivery)
- AiRNA (mRNA design)
- AiTEM (small-molecule formulation)
- Progress:
- Liver-targeted delivery efficiency surpasses industry leaders by 20x.
- Phase III trial underway for a small-molecule drug; mRNA immunotherapy entering clinical trials in 2024.
Signet Therapeutics: FDA IND for First AI + Organoid-Discovered Gastric Cancer Drug

- Breakthrough: Global-first AI/Organoid-derived drug sigx1094 for diffuse gastric cancer secured FDA IND.
- Collaboration: With XtalPi, identified a novel target and advanced from discovery to IND in 3 years.
- Potential: Demonstrates efficacy in ovarian, triple-negative breast, and pancreatic cancers.
META Pharmaceuticals: Rare Pediatric Disease Designation for Kidney Stone Therapy

- FDA Recognition: Granted Rare Pediatric Disease Designation for META-001-PH, an oral therapy reducing urinary oxalate by 80% in preclinical models.
- Timeline: Phase I safety trials in Australia set for H1 2025.
- Value: Eligible for a Priority Review Voucher (PRV) worth ~$100M post-approval.
ReviR Therapeutics: $30M Series A for RNA-Targeted Neurological Therapies

- Focus: Developing oral RNA-targeted therapies for Huntington’s disease, ALS, and CMT.
- Collaboration: Partnered with XtalPi since 2021 to combine VoyageR AI platform with robotic drug discovery.
- Pipeline: Multiple CNS programs advancing to preclinical/clinical stages.