Signet Therapeutics, a company incubated by XtalPi Inc. (2228.HK), has been nominated for the 2025 Prix Galien USA Best Biotechnology Award for SIGX1094R, the world’s first targeted therapy for diffuse gastric cancer, developed in collaboration with XtalPi. Signet Therapeutics is the only Chinese biopharmaceutical company nominated for this prestigious award. The last Chinese biopharma product to receive a Prix Galien nomination was BeiGene’s BTK inhibitor Zanubrutinib.

The 2025 Prix Galien USA Best Biotechnology Award nominees include 16 products from leading global pharmaceutical companies such as Amgen, AstraZeneca, Johnson & Johnson, Pfizer, Merck, and Novartis. SIGX1094R, discovered through the innovative “organoid + AI” platform by Signet Therapeutics and XtalPi, is the first-in-class targeted therapy for diffuse gastric cancer and the first globally to enter clinical trials as a dual FAK/SRC inhibitor. The drug has received Orphan Drug Designation (ODD) and Fast Track Designation (FTD) from the U.S. FDA and is currently undergoing Phase I clinical trials at Peking University Cancer Hospital.
2025 Prix Galien USA Best
Biotechnology Product (Nominees)
| Amgen Inc. | IMDELLTRA® |
| Arcutis Biotherapeutics, Inc. | ZORYVE® |
| AstraZeneca and Sanofi | Beyfortus® |
| Bavarian Nordic | VIMKUNYA™ |
| Catalyst Pharmaceuticals | Agamree® |
| Gilead Sciences | Sunlenca® |
| Johnson & Johnson | TECVAYLI™ |
| Johnson & Johnson | TALVEY™ |
| Johnson & Johnson and Legend Biotech USA Inc. | CARVYKTI® |
| Merck & Co Inc. | CAPVAXIVE™ |
| MGI Tech | DNBSEQ-T20 |
| Novartis Pharmaceuticals Corporation | SCEMBLIX® |
| Pfizer Inc. | ABRYSVO® |
| Pfizer Inc./Astellas | PADCEV® |
| Regeneron and Sanofi | Dupixent® |
| Signet Therapeutics | SIGX1094R |
A Breakthrough in Chinese AI-Driven Drug Discovery
The Prix Galien, established in 1970 and often referred to as the “Nobel Prize of Pharmaceuticals,” is one of the highest honors in the biopharmaceutical industry. It recognizes scientific innovation and the tangible impact of therapies on human health. The Prix Galien USA, launched in 2007, is highly competitive, with awards judged by an esteemed committee. The 2025 committee includes 11 distinguished members, such as Nobel Laureates Linda B. Buck, Stanley B. Prusiner, and Phillip A. Sharp, MIT scientist Robert S. Langer, Stanford University President Emeritus Marc Tessier-Lavigne, and former Gates Foundation CEO Susan Desmond-Hellmann.

Previous winners of the Prix Galien USA over the past two years include Pfizer’s PAXLOVID, the first oral COVID-19 treatment; AstraZeneca and Daiichi Sankyo’s ADC for HER2+ breast cancer, ENHERTU; Novo Nordisk’s type 2 diabetes drug semaglutide; and Bristol Myers Squibb’s Camzyos for obstructive hypertrophic cardiomyopathy—each representing groundbreaking therapeutic advancements.
This nomination underscores the success of XtalPi’s cutting-edge AI drug discovery capabilities combined with Signet Therapeutics’ innovative organoid evaluation technology, earning recognition from top industry experts. It not only validates this innovative pipeline but also highlights the emergence of AI and organoid-driven drug discovery as a transformative force in advancing pharmaceutical innovation.
Notably, XtalPi contributed to the development of 2024 Prix Galien USA winner PAXLOVID, helping Pfizer shorten a months-long research process to just six weeks, enabling PAXLOVID to secure FDA approval one day ahead of competitors and become the world’s first oral COVID-19 treatment. In 2022, PAXLOVID generated $18.9 billion in sales.
The nomination of SIGX1094R, a first-in-class molecule discovered from scratch by XtalPi’s platform, reaffirms the company’s ability to design highly competitive novel drugs and efficiently translate them into clinical candidates, showcasing the reproducible strength of its AI-driven platform.
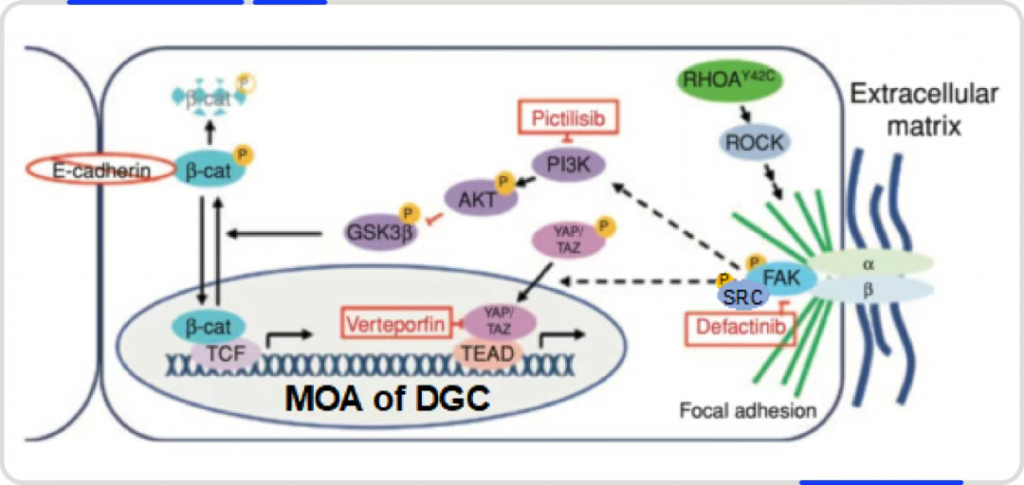
Quantum Physics, AI, and Organoids: Tackling the Dual-Target Challenge in Diffuse Gastric Cancer
Gastric cancer is the fifth most common cancer globally and the fourth leading cause of cancer-related deaths, with approximately 770,000 fatalities annually. Nearly 50% of new gastric cancer cases occur in China, where it ranks fifth in cancer incidence and third in cancer mortality, according to the National Cancer Center.
Addressing this challenge, Signet Therapeutics developed the first diffuse gastric cancer organoid disease model, elucidating the disease’s mechanism and identifying focal adhesion kinase (FAK) as a novel target. These findings were published in Cancer Discovery (2020;10(2):288-305). In collaboration with XtalPi, Signet Therapeutics developed SIGX1094R, the world’s first targeted therapy for diffuse gastric cancer, leveraging the organoid + AI platform. It is also the first dual FAK/SRC inhibitor to enter clinical trials. Remarkably, XtalPi delivered the preclinical candidate (PCC) in just six months, and the project progressed from target discovery to IND approval in just over three years—significantly faster than traditional drug development timelines.
During the discovery and design phase of SIGX1094R, XtalPi utilized its AI and robotics-driven platform to conduct simultaneous target validation and lead compound discovery and optimization, generating a library of FAK inhibitor molecules and identifying those with optimal activity and drug-like properties. However, evaluations in Signet’s diffuse gastric cancer organoid model revealed that the most potent FAK inhibitors did not always yield the best efficacy, showing only partial correlation. This suggested the involvement of additional targets.
Using its proprietary Xpose algorithm to predict molecular binding modes and XFEP (Free Energy Perturbation) algorithm to calculate binding affinities, XtalPi identified SRC, a synergistic protein of FAK, as a new potential target. Experimental validation confirmed the correlation between SRC activity and molecular efficacy, demonstrating for the first time that dual inhibition of FAK and SRC outperforms single-target inhibition. FAK and SRC form a complex that activates downstream pathways, and inhibiting only one allows compensatory activity by the other, reducing antitumor efficacy.
Based on this insight, XtalPi designed a new series of candidate molecules using its AI and robotics platform to simultaneously block FAK and SRC signaling pathways. These molecules demonstrated superior efficacy in organoid models, overcoming the limitations of single-target inhibitors. The study also validated XtalPi’s platform’s robust capabilities in target validation and molecular design, providing critical support for future drug development.
Signet Therapeutics then used its proprietary organoid disease model platform for target validation and efficacy evaluation, confirming SIGX1094R as the clinical candidate for preclinical development. SIGX1094R inhibits both phosphorylated FAK and SRC, blocking the FAK/SRC complex and related signaling pathways. Beyond diffuse gastric cancer, preclinical studies suggest its potential in treating various cancers and in combination therapies (e.g., with KRAS or EGFR inhibitors).
SIGX1094R is currently in Phase I clinical trials at Peking University Cancer Hospital, showing good safety and preliminary antitumor activity in dose-escalation studies. In the second dose cohort (12.5 mg, approximately 1/16 of the target 200 mg dose), a patient with advanced solid tumors and lung metastases achieved stable disease (SD) in two consecutive tumor assessments and has continued treatment for over nine weeks, indicating effective control of advanced malignancies at low doses. SIGX1094R holds significant potential for broader applications, including combination therapies for breast cancer and other tumors. Signet Therapeutics and XtalPi are also advancing multiple innovative cancer-targeted drug discovery projects.
Partnering for Innovation: Scaling AI and Robotics in Drug Discovery
Dr. Zhang Haisheng, Founder of Signet Therapeutics, stated, “We are honored that SIGX1094R, the world’s first organoid + AI-developed drug to enter clinical trials, has been nominated for the Prix Galien. This recognition from top global experts affirms the strength of Signet Therapeutics and XtalPi’s R&D capabilities and validates the organoid + AI model. XtalPi’s cutting-edge target validation and drug design capabilities enabled us to rapidly uncover a novel mechanism for a challenging target and design a highly active, drug-like molecule using Signet’s unique organoid platform, accelerating SIGX1094R’s path to the clinic. We look forward to deepening our collaboration with XtalPi to deliver more breakthrough treatments for patients worldwide.”
Dr. Wen Shuhao, Chairman of XtalPi, added, “SIGX1094R, one of XtalPi’s earliest de novo drug discovery projects, earning recognition from the Prix Galien’s esteemed jury is truly inspiring. As a platform for groundbreaking innovation, XtalPi not only collaborates with top global pharmaceutical companies but also leverages its integrated AI and robotics platform to accelerate the translation of cutting-edge science into clinical assets. We are excited to partner with more innovators to create transformative molecules and unlock the immense potential of AI and automation in biopharmaceuticals, delivering high-value pipelines for global healthcare.”
As the first company listed under Hong Kong’s Chapter 18C and the “AI + Robotics” pioneer, XtalPi has developed an intelligent, autonomous R&D platform integrating quantum physics, AI, and high-precision robotics to advance life sciences and new materials. A leader in AI-driven drug discovery, XtalPi serves 16 of the world’s top 20 pharmaceutical companies. In 2023, XtalPi signed a $250 million AI and robotics drug discovery deal with Eli Lilly, setting a record for China’s largest single-pipeline AI pharma collaboration at the time. In August 2025, XtalPi partnered with DoveTree, founded by biopharma legend Dr. Gregory Verdine, in a $5.99 billion (HKD 47 billion) AI drug discovery deal, breaking the record for the largest AI drug discovery order and receiving an initial payment of HKD 400 million ($51 million). XtalPi is currently supporting dozens of drug discovery projects, several of which are applying for or have received clinical trial approvals.

For more information on past Prix Galien USA winners, visit the official website.