01
Solid state research
Experimental solid-state R&D for the future of drug development
02
Overview
Solid-state research services from pre-clinical to NDA
Our experimental services cover the entire spectrum of solid-state research, guiding projects from pre-clinical stages through to NDA filing. We focus on delivering a seamless and comprehensive drug development process, enhancing pharmaceutical R&D efficiency and ensuring success at every regulatory phase.
Research services
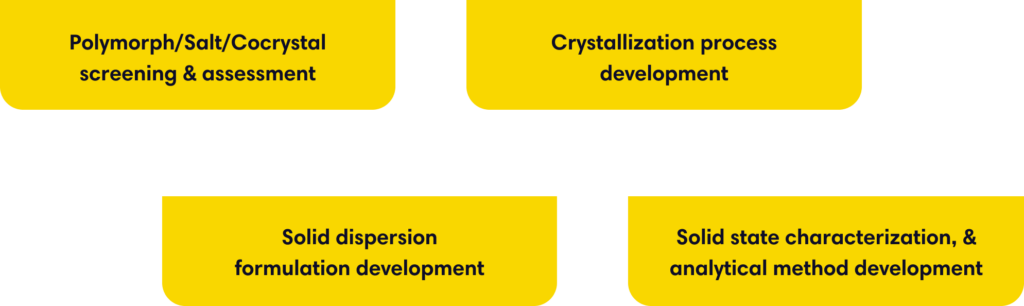
- Polymorph/Salt/Cocrystal screening & assessment
- Crystallization process development
- Solid dispersion formulation development
- Solid state characterization, & analytical method development
03
Automated Solid Form Screening and Selection
R&D with solid-state chemistry screening platform
The intelligent automated solid-state screening platform transforms pharmaceutical R&D by integrating experimental techniques with automation. This approach reduces costs, boosts productivity, and accelerates the research and development process for faster results.



- Application scenarios
Comprehensive polymorph screening identifies and analyzes different crystal forms
Salt and co-crystal screening improves drug stability and solubility
Assessment of solid form developability includes evaluating solubility, stability, and hygroscopicity
Solid form analysis ensures intellectual property protection and supports regulatory submissions
Enhancement of drug bioavailability and key physicochemical properties
04
Crystallization process development
Optimizing drug properties through crystallization process development
Crystallization process development enhances the properties of active pharmaceutical ingredients (APIs) by improving particle-size distribution, crystal morphology, bulk properties, and flowability. These improvements lead to better processability and dissolution of drugs, ensuring more efficient production and enhanced performance.
- Application scenarios
Scenarios 01
Improving Powder Properties
Powder properties are enhanced to improve drug performance and manufacturability.
Scenarios 02
Preparing Meta-Stable Forms
Meta-stable forms are prepared to optimize drug stability and efficacy.
Scenarios 03
Kilogram-Scale Crystallization
Crystallization processes are developed at kilogram scale to meet production demands.
- Key factors process
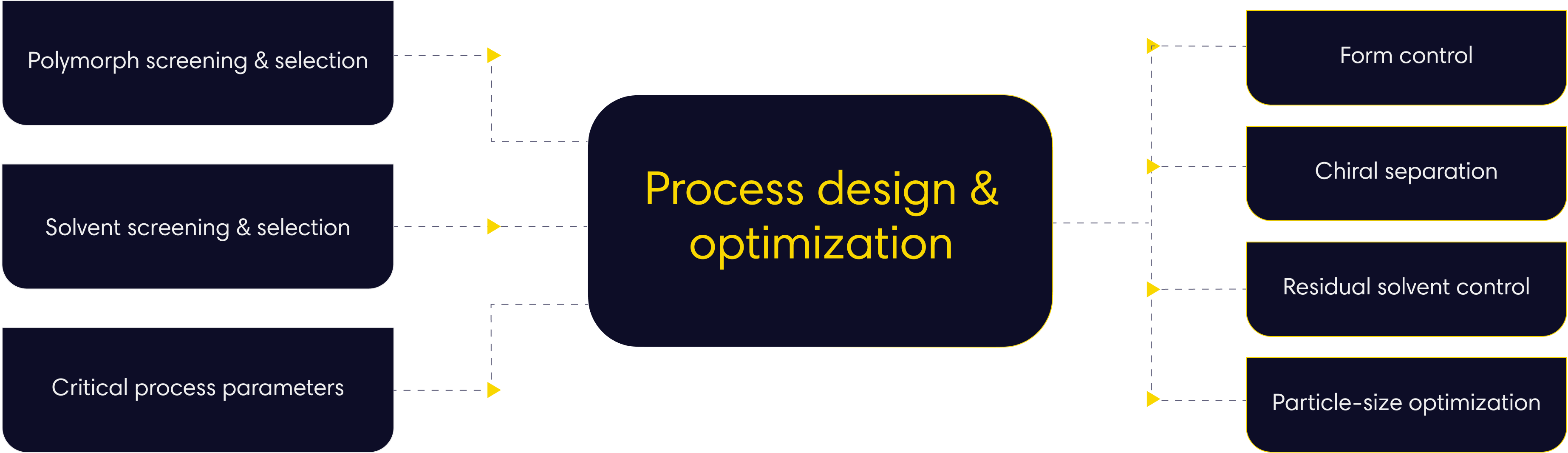
05
Amorphous solid dispersion formulation development
Enhancing drug solubility with amorphous solid dispersion
Technologies like spray drying are used to disperse insoluble drugs in carrier polymer materials, improving solubility. These polymers are carefully screened to ensure the amorphous solid dispersion meets strict stability and dissolution performance standards, even at gram scale. This process enhances drug efficacy and ensures reliable production quality.
- Application scenarios
BCS Class II & IV
drugs require
enhanced solubility
Drugs classified as BCS Class II and IV often have poor solubility, which can limit their effectiveness. Formulation strategies that improve solubility are crucial to enhancing bioavailability and ensuring optimal drug performance.
Solid-state
characterization &
method development
Solid-state characterization and analytical methods ensure that drug formulations meet critical stability, solubility, and performance requirements, optimizing them for both development and manufacturing.
Cutting-edge
testing facilities
State-of-the-art testing supports every stage of solid-state drug development, from particle size analysis to solid form and HPLC chemical analysis, ensuring formulations meet the highest quality standards.
- Solid-State testing overview
Solid-State
characterization methods
This table presents key solid-state testing methods, detailing the time and sample size required. These techniques ensure accurate analysis of drug properties, supporting stability, solubility, and manufacturing efficiency.

Ready to streamline your solid state research?
Contact us today, and discover how XtalGazer can revolutionize your drug discovery process.
